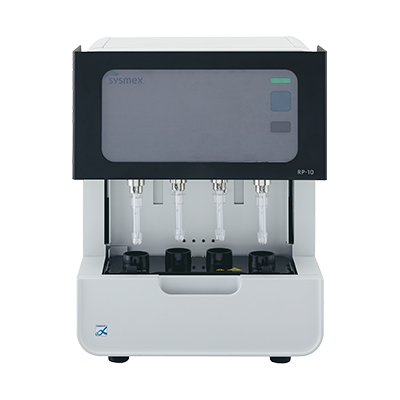
RD-210
OSNA – Advanced platform for analysing lymph nodes
- Short TAT (approx. 15m for 1 sample, approx. 30m for 14 samples)
- High flexibility (multi-application/multi-patient usage)
- Simple reagent management and consumption traceability by QR Code identification
Nodal status matters
More differentiated information – Supporting better treatment decisions
Lymph node status is one of the most important prognostic factors in many cancers and a key criterion for surgical and therapeutic decisions. Analysing lymph node tissue reliably and accuracy is therefore crucial for detecting metastases.
Routine practice in histopathology analyses only a limited amount of tissue and therefore provides only limited information, which might not reflect the real metastatic burden.
OSNA – One Step Nucleic Acid Amplification – is an automated molecular diagnostic assay that analyses the entire lymph node tissue. The reaction is based on rapid nucleic acid amplification technology (RT-LAMP*) to quantify Cytokeratin 19 (CK19) mRNA expression. CK19 is an epithelial cell marker and is not normally present in lymph node tissue. The expression rate of CK19 mRNA correlates with the size of the metastatic foci.
OSNA – Benefits
Superior diagnostic information
- More accurate determination of the metastatic burden by analysing the entire lymph node
- Standardised, objective assessment of the lymph node status
Confidence
- Fast availability of results for optimal decision-making as to the right surgical/ non-surgical approach
- Confident staging as reliable basis for choosing therapy
Patient quality of life
- Next therapy measures can start earlier
- Reduced waiting time and less patient stress
* RT-LAMP = Reverse transcriptase loop-mediated isothermal amplification; licensed under the agreement with Eiken Chemical CO., LTD
Sysmex CZ s.r.o.
Plynárenská 499/1
602 00 Brno
+420 548 216 855
+420 548 216 343
Sysmex Slovakia s.r.o.
Trenčianská 47
821 09 Bratislava
+421 2 6453 2881-2
+421 2 6428 1651
Product documents
Safety data sheets
Regulatory Documents
Regulatory documents, such as Instructions for Use, can be accessed with a valid My Sysmex login:
Go to My Sysmex
![[.CZ-cs Czech Republik (czech)] [.CZ-cs Czech Republik (czech)]](/fileadmin/_processed_/e/c/csm_RD-210_side_view_right_9596ffaebf.png)

![[.CZ-cs Czech Republik (czech)] [.CZ-cs Czech Republik (czech)]](/fileadmin/_processed_/a/3/csm_RD-210_side_view_right_open_5058cc8982.png)
![[.CZ-cs Czech Republik (czech)] [.CZ-cs Czech Republik (czech)]](/fileadmin/_processed_/2/5/csm_OSNA_workflow_58fbc87e34.jpg)
![[.CZ-cs Czech Republik (czech)]](/fileadmin/_processed_/4/4/csm_Workflow_of_OSNA_RD-210_6cb072deba.png)

![[.CZ-cs Czech Republik (czech)] [.CZ-cs Czech Republik (czech)]](/fileadmin/_processed_/e/c/csm_RD-210_side_view_right_0aceb81190.png)

![[.CZ-cs Czech Republik (czech)] [.CZ-cs Czech Republik (czech)]](/fileadmin/_processed_/a/3/csm_RD-210_side_view_right_open_0b4ad9e9c1.png)
![[.CZ-cs Czech Republik (czech)] [.CZ-cs Czech Republik (czech)]](/fileadmin/_processed_/2/5/csm_OSNA_workflow_19806273a9.jpg)